- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement

All Names: Evusheld、Ixagevimab、lgavimab
Indications:Pre-exposure prophylaxis EVUSHELD is indicated for the pre-exposure prophylaxis of COVID-19 in adults and adolescents aged 12 years and older weighing at least 40 kg Treatment EVUSHELD is indicated for the treatment of adults and adolescents (aged 12 years and older weighing at least 40 kg) with COVID-19, who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19
Manufacturer:AstraZeneca,Britain
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Pre-exposure prophylaxis
EVUSHELD is indicated for the pre-exposure prophylaxis of COVID-19 in adults and adolescents aged 12 years and older weighing at least 40 kg
Treatment
EVUSHELD is indicated for the treatment of adults and adolescents (aged 12 years and older weighing at least 40 kg) with COVID-19, who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19
Posology and method of administration
Posology
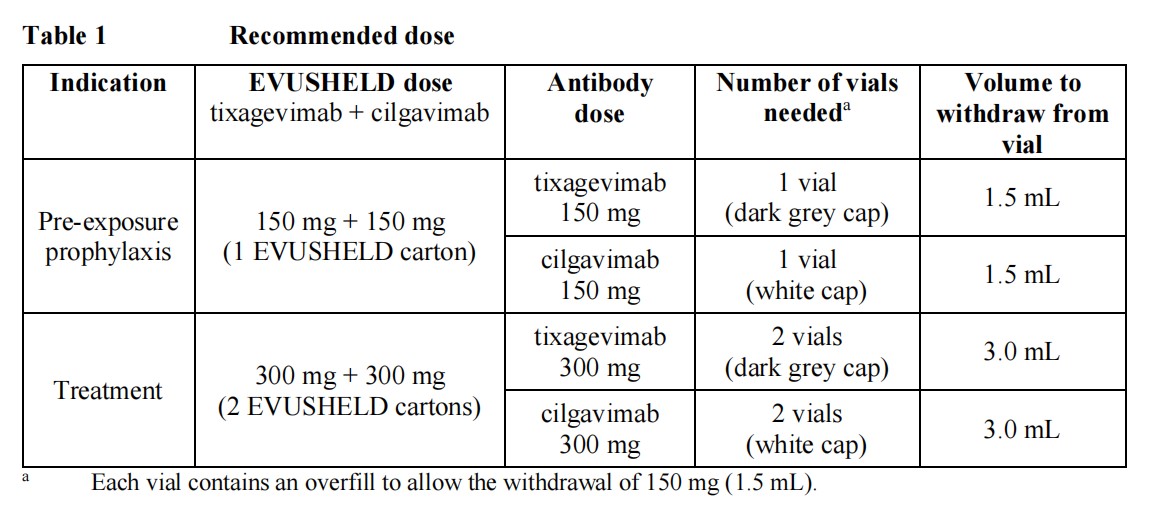
Pre-exposure prophylaxis The recommended dose in adults and adolescents aged 12 years and older weighing at least 40 kg is 150 mg of tixagevimab and 150 mg of cilgavimab, administered as two separate sequentialintramuscular injections.There are no safety and efficacy data available on repeat dosing.TreatmentThe recommended dose in adults and adolescents aged 12 years and older weighing at least 40 kg is 300 mg of tixagevimab and 300 mg of cilgavimab (Table 1), administered as two separate sequential intramuscular injections.EVUSHELD should be given as soon as possible after a positive viral test for SARS-CoV-2 and within 7 days of the onset of symptoms of COVID-19.
The risks and benefits should be considered prior to initiating EVUSHELD in individuals at high risk for cardiovascular or thrombo-embolic events. Patients should be advised of signs or symptoms suggestive of cardiovascular event (notably chest pain, dyspnoea, malaise, feeling lightheaded or faint) and to seek immediate medical attention if such symptoms occur.
Clinically significant bleeding disorders As with any other intramuscular injections, EVUSHELD should be given with caution to patients with thrombocytopenia or any coagulation disorder. Antiviral resistance The clinical trials with EVUSHELD were conducted when Alpha, Beta, Gamma and Delta variants were predominant. Efficacy of tixagevimab and cilgavimab against some circulating SARS-CoV-2 variants with decreased in-vitro susceptibility is uncertain.Based on clinical data from PROVENT, the duration of protection following administration of a single EVUSHELD dose (150 mg of tixagevimab and 150 mg of cilgavimab) is estimated to be at least 6 months. Due to the observed decrease in in-vitro neutralisation activity against the Omicron subvariants BA.1, BA.1.1 (BA.1+R346K), BA.4 and BA.5 the duration of protection of EVUSHELD for these subvariants is currently not known. COVID-19 vaccinesPre-exposure prophylaxis with EVUSHELD is not a substitute for vaccination in individuals for whom COVID-19 vaccination is recommended.
Evusheldinformation
No information yet!!!