- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
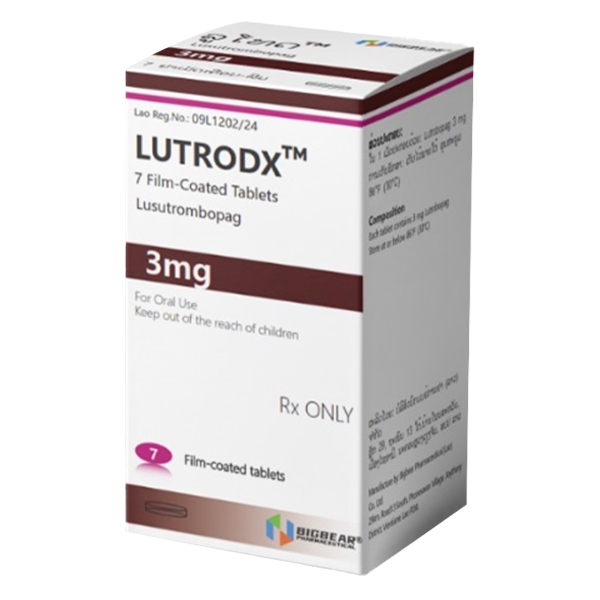
All Names: Mulpleta、lusutrombopag、芦曲泊帕
Indications:Suitable for adult patients with chronic liver disease and planned surgery for thrombocytopenia.
Manufacturer:Daxiong
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Lusatrombopag promotes platelet production and reduces the need for preoperative platelet transfusion by activating TPO receptors on megakaryocytes.
1、 Drug name
Common name: Lu Qu Po Pa
Product Name: MULPLETA ®
2、 Indications
Used to treat thrombocytopenia in adult patients with chronic liver disease who plan to undergo surgery.
3、 Specifications and characteristics
Specification: Each tablet contains 3 milligrams of Luqupopa.
Appearance: A light red circular thin film garment piece, with the Shionogi trademark and code "551" engraved on one side and "3" engraved on the other side.
4、 Main components
Active ingredient: Luqupopa.
Accessories include: D-mannitol, microcrystalline cellulose, magnesium oxide, sodium dodecyl sulfate, hydroxypropyl cellulose, carboxymethyl cellulose calcium, magnesium stearate, hydroxypropyl methylcellulose, triethyl citrate, titanium dioxide, red iron oxide, and talc powder.
5、 Usage and dosage
Recommended dosage: Take 3 milligrams orally once a day for 7 consecutive days.
Medication timing: Medication should be taken 8 to 14 days before the planned surgery. The surgery should be performed within 2 to 8 days after the last administration of medication.
Usage: Can be taken with food or alone.
6、 Dose adjustment
No specific dosage adjustment plan was mentioned for patients with liver and kidney dysfunction. For patients with severe renal or liver dysfunction, data is limited and caution should be exercised when using it.
7、 Medication precautions
It can be taken before and after meals.
If one dose is missed, it should be taken as soon as possible on the same day and the normal medication schedule should be restored the next day. Double doses should not be taken.
During and after medication, platelet count should be closely monitored to prevent excessive platelet elevation and the risk of thrombosis.
This medication is only used to increase platelet count to an acceptable level for surgery and should not be attempted to restore normal platelet count through medication.
8、 Medication for special populations
Pregnancy period: No human data available, animal studies have shown developmental toxicity, and should be used when potential benefits outweigh risks.
Breastfeeding period: It is not recommended to breastfeed during the treatment period and for at least 28 days after the last dose.
Children: Safety and efficacy have not yet been established.
Elderly patients: Clinical studies have not found significant differences in response compared to younger patients.
9、 Adverse reactions
The most common adverse reaction is headache.
Serious risk: Thrombosis/thromboembolic complications, including portal vein thrombosis.
10、 Contraindications
None.
11、 Drug interactions
When used in combination with cyclosporine or antacids containing multivalent Yangzi, no clinically significant changes were observed in the exposure of Luqupopa.
When used in combination with midazolam, no clinically significant changes in midazolam exposure were observed.
In vitro studies have shown that Luqu Po Pa is a substrate for P-gp and BCRP, but has low inhibitory potential for major CYP enzymes and transporters.
12、 Storage method
Stored in original packaging, temperature controlled between 20 ° C and 25 ° C, allowing for short distance transportation between 15 ° C and 30 ° C.
It should be placed out of reach of children.
Lusutrombopaginformation