- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
All Names: Regorafenib、Stivarga、瑞格非尼、瑞戈非尼、瑞格菲尼、拜万戈
Indications:Patients with colorectal cancer, gastrointestinal stromal tumors, and hepatocellular carcinoma who have received certain treatments in the past.
Manufacturer:LUCIUS PHARMACEUTICAL (LAOS) CO., LTD
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Regorafenib is a multi kinase inhibitor that blocks the proliferation and spread of tumor cells by targeting various kinases related to tumor angiogenesis, tumorigenesis, and metastasis, such as VEGFR1-3, KIT, RET, PDGFR, etc.
1、 Drug name
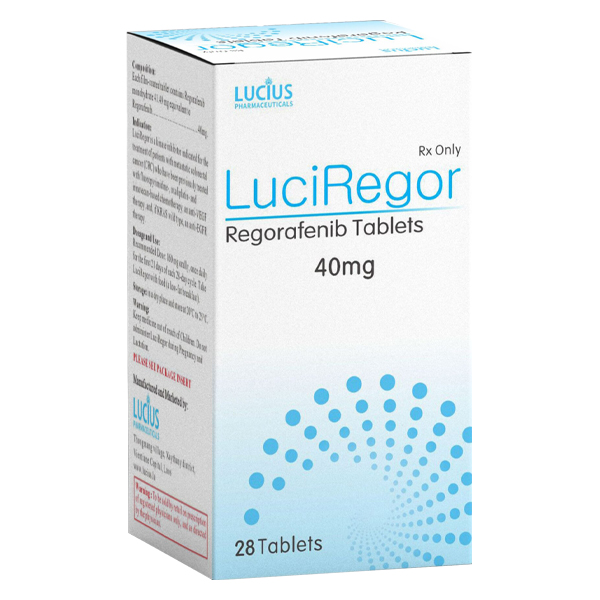
1. Common name: Regorafenib
2. Product Name: STIVARGA
2、 Indications
Used to treat patients with metastatic colorectal cancer who have previously received chemotherapy based on fluorouracil, oxaliplatin, and irinotecan, anti VEGF therapy, and anti EGFR therapy (KRAS wild-type).
3、 Specifications and characteristics
40mg film coated tablets.
4、 Main components
1. Active ingredient: Regorafenib monohydrate (each tablet contains 40mg of Regorafenib anhydrous).
2. Accessories: Microcrystalline cellulose, cross-linked carboxymethyl cellulose sodium, magnesium stearate, polyvinylpyrrolidone, colloidal silica, etc.
5、 Usage and dosage
1. Recommended dosage: 160mg (4 tablets) orally, once daily, taken continuously for 21 days and stopped for 7 days (28 days per cycle).
2. Usage: It should be taken together with a low-fat breakfast (fat content<30%) and swallowed whole. If missed, it cannot be replenished on the same day.
6、 Dose adjustment
1. Reduce to 120mg: After the first occurrence of grade 2 hand foot skin reaction (HFSR) or any grade 3/4 adverse reactions have recovered.
2. Reduction to 80mg: grade 2 HFSR or grade 3/4 adverse reactions occurred again at a dose of 120mg (excluding hepatotoxicity).
3. Permanent discontinuation: Inability to tolerate a dose of 80mg, severe liver toxicity (such as ALT/AST>20 times the upper limit of normal), or grade 4 adverse reactions.
7、 Medication precautions
1. Dietary requirements: Must be taken with a low-fat breakfast (such as two slices of white toast+low-fat jam+skim milk).
2. Omission treatment: Cannot be replenished, continue taking medication according to the original plan the next day.
3. Vomiting treatment: If vomiting occurs after taking medication, there is no need to take additional medication.
4. Before and after surgery: Stop medication for at least 2 weeks before surgery, and evaluate whether to resume medication after the wound has healed.
8、 Medication for special populations
1. Pregnant women: Prohibited, may cause fetal malformation (pregnancy grade D).
2. Breastfeeding period: Stop taking medication or breastfeeding.
3. Liver/kidney dysfunction: No dose adjustment is required for mild to moderate cases, and it is not recommended for severe liver dysfunction (Child Pugh C).
4. Elderly: No need to adjust dosage.
9、 Adverse reactions
1. Common (≥ 30%) symptoms include fatigue, decreased appetite, skin reactions on hands and feet, diarrhea, stomatitis, weight loss, infection, hypertension, and hoarseness.
2. Serious adverse reactions may include liver toxicity, bleeding, gastrointestinal perforation, etc.
10、 Contraindications
There are no clear absolute contraindications, but it is contraindicated for those who are allergic to the drug ingredients.
11、 Drug interactions
1. Avoid co administration: Strong CYP3A4 inducers (such as rifampicin) or inhibitors (such as ketoconazole) may affect drug efficacy or toxicity.
2. Anticoagulants: Close monitoring of INR is required when combined with warfarin.
12、 Storage method
Store at room temperature (20-25 ° C), keep away from moisture and light, and maintain the original packaging.
Regorafenibinformation