- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
All Names: Xanomeline and Trospium Chloride、Cobenfy、KarXT、呫诺美林曲司氯铵胶囊
Indications:Used for treating adult schizophrenia.
Manufacturer:Bristol-Myers Squibb Company,USA
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Cobenfy is an oral capsule formulation composed of Xanomeline (a muscarinic receptor agonist) and Trospiumchloride (a muscarinic receptor antagonist).
1、 Drug name
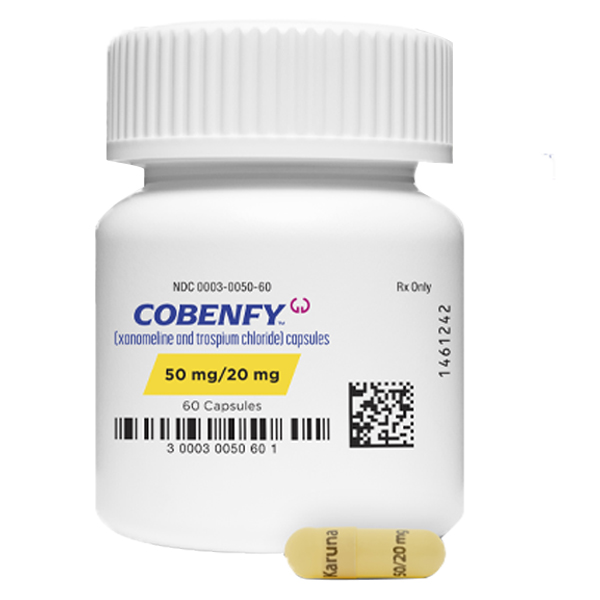
COBENFY ™ (xanomelineandtrospiumchloride)capsules, In Chinese, it can be called Cobenfy (KarXT).
2、 Indications
Used for treating adult schizophrenia.
3、 Specifications and characteristics
This product is a capsule with three specifications:
1. 50mg/20mg (xanomiline/rospium chloride): a light yellow capsule with the label "Karuna 50/20mg".
2. 100mg/20mg (xanomiline/rospium chloride): Brown capsules with the words "Karuna 100/20mg" printed on them.
3. 125mg/30mg (xanomiline/rospium chloride): Swedish orange capsules with the label "Karuna 125/30mg".
4、 Main components
Active ingredients: Xanomeline (a muscarinic agonist) and Trospiumchloride (a muscarinic antagonist).
5、 Usage and dosage
1. Recommended starting dose: 50mg/20mg capsules, taken orally, twice daily, for at least 2 days.
2. Dose escalation: then increase to 100mg/20mg capsules orally, twice daily, for at least 5 days.
3. Maintenance/maximum dose: Depending on patient tolerance and response, the dose can be increased to 125mg/30mg capsules orally, twice daily. The maximum recommended dose is 125mg/30mg, twice daily.
4. Medication time: It should be taken at least 1 hour before meals or at least 2 hours after meals. Please do not open the capsule.
6、 Dose adjustment
1. Elderly patients: The recommended starting dose is 50mg/20mg capsules, taken orally twice a day. Slow dose escalation should be considered. The maximum recommended dose is 100mg/20mg capsules, twice daily.
2. Patients with renal insufficiency:
Mild renal insufficiency (eGFR60 to<90mL/min): The recommended dosage is the same as for patients with normal renal function.
Moderate or severe renal insufficiency (eGFR<60mL/min): Not recommended for use.
3. Patients with liver dysfunction:
Mild liver dysfunction (Child Pugh A grade): Not recommended for use.
Moderate or severe liver dysfunction (Child Pugh B or C): contraindicated.
7、 Medication precautions
1. Before and after meals: It must be taken on an empty stomach, at least 1 hour before meals or at least 2 hours after meals.
2. Missed dose: Not explicitly stated, the usual principle should be followed: if it is close to the next dose, skip the missed dose and take the next dose at the regular time; Do not double the dosage. Please consult a doctor or pharmacist for specific information.
3. Vomiting: If vomiting occurs after taking medication, it is not clearly stated whether to take it again. If this situation occurs, you should consult a doctor.
4. General precautions:
Before and during treatment, liver enzymes, bilirubin, and heart rate should be evaluated according to clinical indications.
May cause dizziness, drowsiness, hallucinations, or blurred consciousness. Before determining the impact of this product on oneself, one should avoid driving or operating heavy machinery.
Notify the doctor of all medications currently in use (including prescription drugs, over-the-counter drugs, vitamins, and herbal supplements) as there may be interactions.
8、 Medication for special populations
1. Pregnancy period: There is currently no data on the use of drugs by pregnant women. Suggest informing the doctor if you are pregnant or planning to become pregnant.
2. Breastfeeding period: It is not yet clear whether active ingredients are secreted with human milk. The developmental and health benefits of breastfeeding for infants, as well as the clinical needs of mothers for COBENFY and the potential impact of drugs on infants, should be considered.
3. Children: Safety and efficacy have not yet been established.
4. Elderly patients: refer to the "Dose Adjustment" section. Increased risk of urinary retention.
5. Renal insufficiency: Refer to the "Dose Adjustment" section. The risk of anticholinergic adverse reactions increases.
6. Liver dysfunction: Refer to the "Dose Adjustment" section. The risk of adverse reactions increases.
9、 Adverse reactions
1. Common adverse reactions (with an incidence rate ≥ 5% and at least twice that of the placebo group): nausea, indigestion, constipation, vomiting, hypertension, abdominal pain, diarrhea, tachycardia, dizziness, and gastroesophageal reflux disease.
2. Serious Adverse Reaction Warning:
Risk of urinary retention: especially in elderly patients and patients with bladder outlet obstruction. Symptoms such as difficulty urinating, weak urine flow, incomplete urination, and painful urination need to be monitored.
Risk of liver injury: contraindicated for patients with moderate to severe liver injury, not recommended for patients with mild liver injury. Liver enzymes need to be monitored.
Risk of vascular edema: It may occur in the face, lips, tongue, and throat, and in severe cases, it can be life-threatening.
Increased heart rate: can lead to an increase in heart rate.
Central nervous system effects: including dizziness, confusion, hallucinations, and drowsiness.
Gastrointestinal motility decline: Use with caution in patients with gastrointestinal obstructive diseases.
Narrow angle glaucoma risk: Only for patients with known anatomical narrow angles when the benefits outweigh the risks, and close monitoring is required.
10、 Contraindications
This product is contraindicated for the following patients:
1 Urinary retention.
2. Moderate or severe liver injury.
3. Gastric retention.
4. Have a history of allergies to COBENFY, trospiumchloride, or any ingredients.
5. Untreated narrow angle glaucoma.
11、 Drug interactions
1. Strong CYP2D6 inhibitors: may increase the blood concentration of xanomeline, thereby increasing the risk of adverse reactions and requiring monitoring.
2. Drugs actively secreted and excreted through renal tubules: may compete for excretion pathways, increase blood drug concentrations and the risk of adverse reactions for both parties, and require monitoring.
3. Sensitive substrates of CYP3A4 or P-glycoprotein (oral): may increase the blood drug concentration and risk of adverse reactions due to local inhibition, and need to be monitored.
4. Other anti muscarinic drugs: may increase the frequency or severity of anticholinergic adverse reactions (such as dry mouth, constipation) and need to be monitored.
12、 Storage method
Store at 20 ° C to 25 ° C (68 ° F to 77 ° F); Allow short distance transportation within the range of 15 ° C to 30 ° C (59 ° F to 86 ° F).
13、 Manufacturer
Sold by Bristol Myers Squibb Company.
KarXTinformation
No information yet!!!