- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
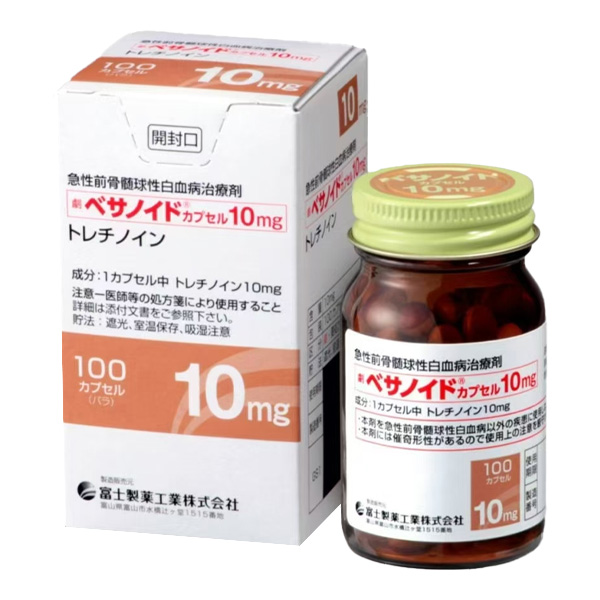
All Names: Tretinoin、Vesanoid、 维甲酸胶囊、全反式维A酸
Indications:Acute promyelocytic leukemia (APL) patients in adults and children aged ≥ 1 year, who require detection of t (15; 17) chromosome translocation or PML/RAR α gene expression, and are suitable for individuals who are resistant to anthracycline chemotherapy, have relapsed, or cannot use anthracycline drugs due to contraindications.
Manufacturer:Fuji Pharmaceutical Co., Ltd., Japan
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Tretinoin is an all trans retinoic acid oral capsule preparation, belonging to the class of vitamin A compounds.
1、 Drug name
Common name: Tretinoin
Product Name: VESANOID ®
2、 Indications
Used to induce remission of acute promyelocytic leukemia (APL) in adults and children aged 1 year and above, suitable for patients who meet the following conditions:
There is a t (15; 17) chromosome translocation or PML/RAR α gene expression.
Resistance or recurrence to anthracycline chemotherapy, or contraindications to anthracycline chemotherapy.
3、 Specifications and characteristics
Specification: Each capsule contains 10mg of retinoic acid.
4、 Main components
Active ingredient: Retinoic acid (all trans retinoic acid).
5、 Usage and dosage
Recommended dose: 22.5mg/m2, orally, twice daily until complete remission is achieved.
The treatment should be stopped 30 days after achieving complete remission or 90 days of total treatment, whichever comes first.
Medication method: Swallow the whole pill, do not chew, dissolve or open the capsule. Should be taken with meals.
6、 Dose adjustment
If moderate or severe differentiation syndrome occurs, it should be considered to suspend medication until symptoms improve.
If intracranial hypertension occurs, interruption, reduction or discontinuation of medication may be considered.
When the liver function indicators increase to more than 5 times the upper limit of normal values, it should be considered to suspend medication until recovery.
If severe or intolerant adverse reactions occur in pediatric patients, dose reduction may be considered.
7、 Medication precautions
Before and after meals: It is recommended to take it with meals.
Missed medication: If it is more than 10 hours before the next medication, it can be supplemented; Otherwise, skip and take the medication according to the original plan.
Vomiting: If vomiting occurs after taking medication, there is no need to take the next dose according to the original plan.
Other: During medication, avoid using vitamin A, other drugs that can cause intracranial hypertension (such as tetracyclines), and anti fibrinolytic drugs.
8、 Medication for special populations
Pregnant women: prohibited, with embryo fetal toxicity.
Breastfeeding period: It is not recommended to breastfeed during the treatment period and within one week after the last dose.
Women of childbearing age: Before treatment, it is necessary to confirm that they are not pregnant. During the treatment period and one month after the last dose, two effective contraceptive measures should be taken.
Male of childbearing age: Effective contraceptive measures should be taken during the treatment period and one week after the last dose.
Children: Available for ages 1 and above, safety has not been established for ages 1 and below.
Elderly patients: There is no significant difference in safety and effectiveness compared to younger patients.
9、 Adverse reactions
Common (≥ 30%) symptoms include headache, fever, dry skin/mucous membranes, bone pain, fatigue, chills, upper respiratory tract infections, difficulty breathing, bleeding, infection, nausea/vomiting, rash, peripheral edema, leukocytosis, pain, gastrointestinal bleeding, chest discomfort, and abdominal pain.
Serious adverse reactions include differentiation syndrome (about 26%), rapid increase in white blood cells (about 40%), intracranial hypertension, dyslipidemia, hepatotoxicity, thromboembolic events, etc.
10、 Contraindications
Prohibited for individuals allergic to retinoic acid, any excipients, or other retinoid drugs.
11、 Drug interactions
Avoid co administration with potent CYP3A inhibitors or inducers.
Avoid co administration with drugs that can cause intracranial hypertension.
Avoid co administration with vitamin A.
Avoid co administration with anti fibrinolytic drugs.
12、 Storage method
Stored at 20 ° C-25 ° C, short-term storage at 15 ° C-30 ° C is allowed.
Avoid light and tightly seal the bottle cap.
Tretinoininformation