- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
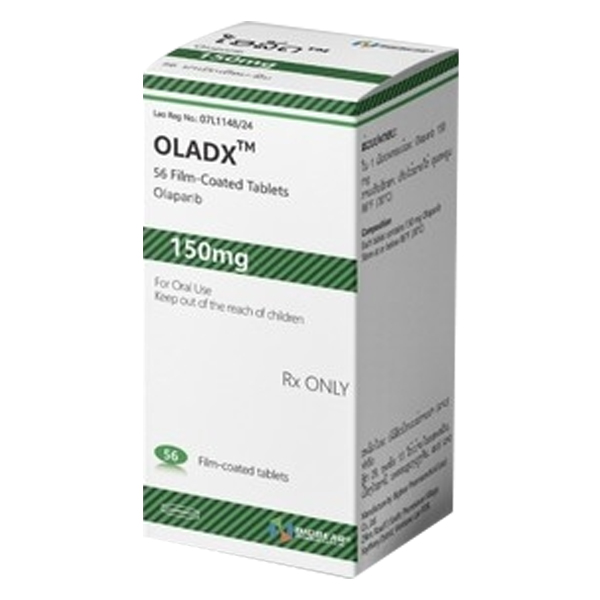
All Names: Olaparib、Lynparza、Olanib、Olaparix、Lynib、奥拉帕尼、奥拉帕利、利普卓
Indications:Adult patients with advanced ovarian cancer of BRCA with germline mutation, and metastatic breast cancer with germline BRCA gene mutation and HER2 negative, who have previously received chemotherapy including neoadjuvant, adjuvant or targeted at metastatic breast cancer.
Manufacturer:Daxiong
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
On August 23, 2018, the National Medical Products Administration (CFDA) officially approved Olaparib for sale in China, becoming the first PARP inhibitor to be marketed domestically and already included in medical insurance reimbursement.
1、 Drug name
1. Common name: Olaparil
2. Product Name: LYNPARZA ®
3. Dosage form: Tablets
2、 Indications
1. Used for maintenance therapy in adult patients with recurrent epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who fully or partially respond to platinum based chemotherapy.
2. Used to treat adult patients with advanced ovarian cancer carrying germline BRCA mutations (gBRCAm) who have previously received third line or higher chemotherapy. Before use, BRCA mutation status must be confirmed through FDA approved companion diagnostic testing.
3、 Specifications and characteristics
150mg tablets.
4、 Main components
1. Active ingredient: Olaparib.
5、 Usage and dosage
1. Recommended dosage: 300mg each time (2 150mg tablets), taken orally twice a day, with an interval of about 12 hours between morning and evening.
2. Course of treatment: Treatment should be continued until the disease progresses or unacceptable toxicity occurs.
3. Usage: Swallow the whole tablet, do not chew, crush, dissolve or break it open. Can be taken before or after meals.
6、 Dose adjustment
1. Adverse reaction dose adjustment: first reduced to 250 milligrams (1 tablet 150 milligrams+1 tablet 100 milligrams) twice daily; If further reduction is required, reduce to 200 milligrams (2 tablets of 100 milligrams) twice daily.
2. Co administration with CYP3A inhibitors: Co administration should be avoided. If potent CYP3A inhibitors must be used in combination, Olaparib should be reduced to 100 milligrams twice daily; If using intermediate acting CYP3A inhibitors, reduce to 150 milligrams twice daily.
3. Patients with renal insufficiency: The recommended dose for patients with moderate renal impairment (CLcr31-50mL/min) is 200mg twice daily. Patients with mild renal impairment do not need to adjust the dosage. Data on patients with severe renal impairment is missing.
7、 Medication precautions
1. Missed dose: should not be supplemented, the next dose should be taken at the regular time.
2. Vomiting: If vomiting occurs after taking medication, it should not be taken again, and the next dose should be taken at the regular time.
3. Food and Grapefruits: During medication, avoid consuming grapefruit, grapefruit juice, Seville oranges, and Seville orange juice as they may inhibit CYP3A enzymes and increase olaparib blood drug concentrations.
Formulation difference: Tablets and capsules have different bioavailability, and it is strictly prohibited to use them interchangeably based on milligrams.
8、 Medication for special populations
1. Pregnant women: This product can cause fetal damage and is contraindicated for pregnant women. Women of childbearing age should undergo a pregnancy test before starting treatment.
2. Women of childbearing age: Effective contraceptive measures should be taken during treatment and at least 6 months after the last dose.
3. Breastfeeding women: Breastfeeding is prohibited during the treatment period and within one month after the last dose.
4. Children: Safety and efficacy have not yet been established.
5. Elderly patients: No overall differences were observed in clinical studies compared to younger patients.
6. Liver injury patients: Mild liver injury patients do not need to adjust the starting dose. Data on patients with moderate to severe liver injury is missing.
9、 Adverse reactions
1. Common adverse reactions (incidence ≥ 20%) include anemia, nausea, fatigue (including fatigue), vomiting, nasopharyngitis/upper respiratory tract infection/influenza, diarrhea, joint pain/muscle pain, taste disorders, headache, indigestion, decreased appetite, constipation, and stomatitis.
2. Common laboratory abnormalities (incidence ≥ 25%) include: decreased hemoglobin, increased mean red blood cell volume, decreased lymphocytes, decreased white blood cells, decreased absolute neutrophil count, increased serum creatinine, and decreased platelets.
3. Serious Warning:
Myelodysplastic syndrome/acute myeloid leukemia: incidence<1.5%, mostly fatal. Blood routine needs to be monitored.
Pneumonia: incidence rate<1%, partially fatal. New or worsening respiratory symptoms require interruption of treatment and evaluation.
10、 Contraindications
None.
11、 Drug interactions
1. CYP3A inhibitors: Avoid co administration with potent or moderate CYP3A inhibitors, otherwise adjust the dose of olaparib.
2. CYP3A inducers: Avoid co administration with potent or moderate CYP3A inducers as they may reduce the efficacy of olaparib.
3. Antitumor drugs: Co administration with other anti-tumor drugs (especially DNA damaging agents) that have bone marrow suppressive effects may enhance and prolong bone marrow suppressive toxicity.
12、 Storage method
1. Store at 20 ° C to 25 ° C, allowing fluctuations between 15 ° C and 30 ° C.
2. Store in original bottles to prevent moisture.
Olaparibinformation