- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
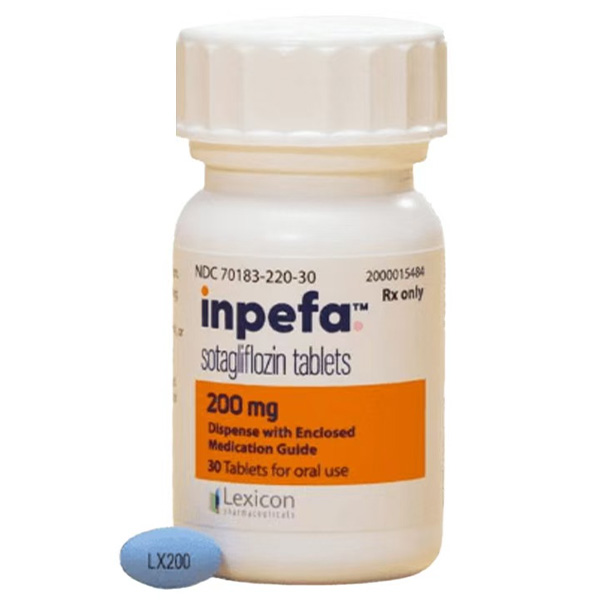
All Names: sotagliflozin、Inpefa、索格列净
Indications:It is applicable to reduce the risk of cardiovascular death, heart failure hospitalization and emergency heart failure consultation for adult patients with heart failure or adult patients with type 2 diabetes related chronic kidney disease and other cardiovascular risk factors.
Manufacturer:美国Lexicon Pharmaceuticals
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Soragliflozin is the first FDA approved dual target inhibitor of SGLT1 and SGLT2. Its function is to inhibit the expression of SGLT1 and SGLT2, thereby achieving the goal of controlling blood sugar and reducing obesity.
1、 Drug name
1. Common name: Sotagliflozin
2. Product Name: INPEFA ™
2、 Indications
Used to reduce the risk of cardiovascular death, hospitalization due to heart failure, and emergency heart failure visits in the following adult patients:
1. Heart failure.
2. Type 2 diabetes with chronic kidney disease and other cardiovascular risk factors.
3、 Specifications and characteristics
The 200mg tablet is an oval shaped, blue film coated tablet with black "LX200" printed on one side.
4、 Main components
Active ingredient: Soragliflozin.
5、 Usage and dosage
1. Usage: Oral administration, once daily. Swallow the whole piece, do not chew, crush or cut open.
2. Dosage: The recommended starting dose is 200mg, once daily. At least 2 weeks later, depending on tolerance, it can be increased to 400mg once daily. It can also be lowered to 200mg as needed.
3. Medication time: It should be taken no more than 1 hour before the first meal of the day.
6、 Dose adjustment
1. Upregulation: Starting at 200mg, for those with good tolerance, it can be upregulated to 400mg at least 2 weeks later.
2. Downregulation: If intolerance (such as adverse reactions) occurs, it can be downregulated to 200mg.
3. Before surgery: If possible, stop taking medication for at least 3 days before major surgery or operations that require prolonged fasting.
4. Patients with decompensated heart failure: Medication should be started immediately after hemodynamic stabilization (including hospitalization, emergency outpatient treatment, or discharge).
7、 Medication precautions
1. Before/after meals: Take before meals. It is recommended to take it no more than 1 hour before the first meal of the day to reduce the impact on postprandial blood sugar.
2. Missed dose: If missed for more than 6 hours, the dose should be skipped and the next dose should be taken at the regular time the next day. Do not take double the dose at once.
3. Vomiting/Diarrhea: If a large amount of body fluids are lost (dehydrated) due to vomiting, diarrhea, or other reasons, one should be alert to the risks of low blood volume and low blood pressure, replenish fluids in a timely manner, and seek medical attention.
4. Laboratory monitoring: Due to its mechanism of action, urine glucose testing may be positive during medication, and urine glucose testing cannot be used to monitor blood sugar. Other methods (such as blood glucose meters) should be used to monitor blood glucose.
8、 Medication for special populations
1. Pregnant women: Based on animal data, it is not recommended to use it in the middle and late stages of pregnancy. Early pregnancy medication needs to be weighed for its pros and cons.
2. Breastfeeding women: Medications may enter breast milk and pose a risk to the development of the newborn's kidneys. Not recommended for use during lactation.
3. Elderly individuals: There is no need to adjust the dosage, but the risk of adverse reactions related to insufficient blood volume (such as hypotension) is higher.
4. Individuals with renal insufficiency:
Patients with mild to moderate renal insufficiency need to adjust the dosage, starting at 200mg and cautiously increasing it.
EGFR below 25mL/min/1.73m ² or in dialysis patients, lacking safety and efficacy data.
5. Patients with liver dysfunction:
Mild liver dysfunction requires dose adjustment.
Patients with moderate or severe liver dysfunction are not recommended to use (drug exposure significantly increases, safety and efficacy have not been established).
6. Children: The safety and efficacy of patients under 18 years old have not been established.
9、 Adverse reactions
1. The most common adverse reactions (incidence rate ≥ 5%) are urinary tract infection, insufficient blood volume, diarrhea, and hypoglycemia.
2. Serious adverse reactions/warnings:
Ketoacidosis in patients with type 1 diabetes and other high-risk patients (may occur when blood sugar is normal).
Insufficient blood volume and symptomatic hypotension, especially in elderly individuals, those with renal insufficiency, or those using diuretics.
Severe urinary tract infections (including urinary sepsis and pyelonephritis).
The risk of hypoglycemia increases when used in combination with insulin or insulin secretagogues.
Necrotizing fasciitis of the perineum (Fournier gangrene).
Genital fungal infection.
Laboratory indicator impact: In the early stages of treatment, there may be a transient increase in blood creatinine and a transient decrease in estimated glomerular filtration rate.
10、 Contraindications
Patients with a history of severe allergic reactions to any component of soragliflozin or INPEFA.
11、 Drug interactions
1. Digoxin: When used in combination, the exposure to digoxin increases, and digoxin levels need to be monitored.
2. UGT enzyme inducers (such as rifampicin): can reduce the net exposure of sorafenib, which may affect efficacy, and clinical monitoring is necessary.
3. Lithium supplements: When used in combination, it may reduce serum lithium concentration, and more frequent monitoring of blood lithium concentration is required during the initiation of INPEFA or dose adjustment.
12、 Storage method
1. Store at room temperature of 20 ° C to 25 ° C (68 ° F to 77 ° F), and allow for brief storage in an environment of 15 ° C to 30 ° C (59 ° F to 86 ° F).
2. Please keep out of reach of children.
13、 Manufacturer
Produced by Lexicon Pharmaceuticals, Inc. (Woodlands, Texas, USA).
Inpefainformation
No information yet!!!