- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
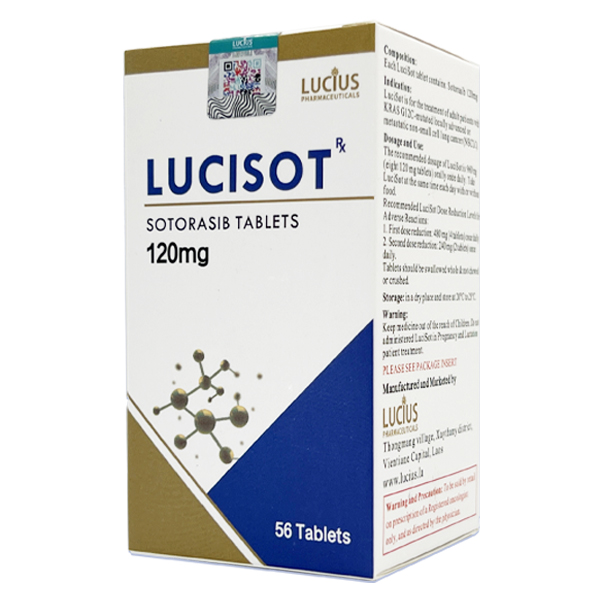
All Names: Sotorasib,LUMAKRAS,AMG510,索托拉西布
Indications:It is indicated for adult patients with locally advanced or metastatic non-small cell lung cancer who have been confirmed to have KRAS G12C mutations by FDA-approved testing and have received at least one systemic treatment, as well as adult patients with metastatic colorectal cancer who have been confirmed to have KRAS G12C mutations by FDA-approved testing and have received chemotherapy with fluoropyrimidines, oxaliplatin, and irinotecan.
Manufacturer:LUCIUS PHARMACEUTICAL (LAOS) CO., LTD
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Drug instructions for Sotorasib/LUMAKRAS
1. Indications
Sotarsib is indicated for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have a KRAS G12C mutation confirmed by an FDA-approved test method. These patients must have previously received at least one systemic treatment (such as a PD-1/PD-L1 inhibitor or platinum-based chemotherapy).It is also used in combination with panitumumab for the treatment of adult patients with KRAS G12C mutant metastatic colorectal cancer (mCRC).
This indication has been granted accelerated approval based on objective response rate (ORR=36%) and duration of response (DOR=10 months), and subsequent confirmatory trials are needed to confirm clinical benefit.
II. Usage and Dosage
The standard dosing regimen is to take 960mg (8 tablets of 120mg specification) orally once daily. The tablets should be swallowed whole and must not be chewed or crushed. It can be taken before or after meals. If a dose is missed for more than 6 hours, skip that dose and continue with the original schedule the next day.
For patients with swallowing difficulties, the tablets can be dispersed in 120mL of non-carbonated room temperature water and stirred until broken (not completely dissolved). The medication should be consumed within 2 hours, and an additional 120mL of water should be used to rinse out any residue from the container.
III. Dose Adjustment
In case of hepatotoxicity (elevated AST/ALT), medication should be suspended until it returns to Grade ≤1. The initial dosage reduction should be to 480mg/day, and the second reduction to 240mg/day. Suspected interstitial lung disease (ILD) should result in immediate suspension of medication, and permanent discontinuation if confirmed. For Grade ≥3 diarrhea/nausea/vomiting or other Grade ≥3 adverse reactions, medication should be suspended until symptoms are alleviated to Grade ≤1, then the dosage should be reduced by one grade for continued treatment. The maximum allowable number of dosage reductions is two, with a minimum dose of 240mg/day.
IV. Common side effects
Adverse reactions with an incidence rate of ≥20% include digestive system symptoms such as diarrhea (42%), nausea (26%), and vomiting (17%), musculoskeletal pain (35%), and general symptoms such as fatigue (26%), as well as liver toxicity-related elevated transaminases (25%) and cough (20%). The main laboratory abnormalities are lymphopenia (48%), decreased hemoglobin (43%), and elevated AST (39%)/ALT (38%).
V. Important Notes
During treatment, ALT/AST/total bilirubin levels need to be monitored every 3 weeks for 3 months, and then once a month. Immediate medical attention should be sought in case of jaundice, darkened urine, or unexplained fatigue. New cough, fever, or difficulty breathing require urgent evaluation for ILD risk. It is prohibited to use strong CYP3A4 inducers (such as rifampin) in combination with PPIs/H2 receptor antagonists (combination must be separated by 4/10 hours), and caution should be exercised when using CYP3A4/P-gp substrates (such as midazolam, digoxin). Breastfeeding is prohibited during medication use and for 1 week after discontinuation in lactating patients. There is no safety data for patients with Child-Pugh B/C liver damage. During treatment, live vaccines should be avoided, and patients of childbearing age need to take effective contraceptive measures.
VI. Drug storage
This product is a yellow film-coated tablet (labeled "AMG120") with a dosage of 120mg per tablet. The packaging specification is 240 tablets per bottle or 120 tablets x 2 bottles. It should be stored at room temperature between 20-25°C and protected from moisture.
Sotorasibinformation