- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
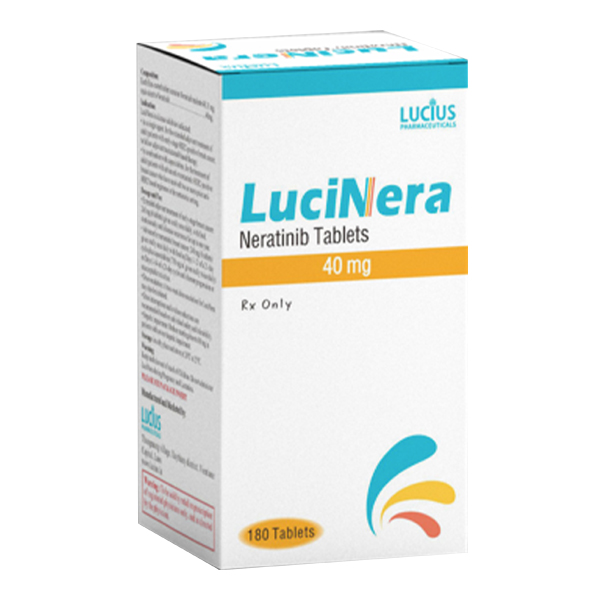
All Names: Hernix、Niratinib、Nerlynx、来那替尼、贺俪安
Indications:Patients with early breast cancer and advanced or metastatic breast cancer.
Manufacturer:LUCIUS PHARMACEUTICAL (LAOS) CO., LTD
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Neratinib was approved by FDA on February 25, 2020 to be used in combination with capecitabine to treat patients with advanced or metastatic HER2 positive breast cancer, provided that the patient has received at least two anti HER2 treatments in the context of metastasis.
1、 Drug name
1. Common name: Neratinib
2. Product Name: NERLYNX ®
2、 Indications
For extended adjuvant treatment of early adult HER2 overexpression/amplification breast cancer, it should be used after adjuvant treatment with trastuzumab.
3、 Specifications and characteristics
1. Tablets: 40mg (equivalent to 48.31mg of Lenvatinib Maleate).
2. Appearance: Film coated tablets.
4、 Main components
1. Active ingredient: Lenvatinib (tyrosine kinase inhibitor)
2. Accessories: colloidal silica, mannitol, microcrystalline cellulose, cross-linked polyvinylpyrrolidone, polyvinylpyrrolidone, magnesium stearate, purified water (core); Polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc powder, iron oxide red (coating).
5、 Usage and dosage
1. Recommended dose: 240mg (6 tablets) once daily, taken orally with meals, for continuous use for 1 year.
2. Diarrhea prevention: Loperamide should be used from the first administration for the first 2 cycles (56 days), with 1-2 bowel movements per day maintained.
4. Omission treatment: skip the daily dose and take the medication according to the original plan the next day.
6、 Dose adjustment
1. Diarrhea management:
Grade 1 or Grade 2 diarrhea (<5 days): Adjust antidiarrheal medication and maintain fluid replacement.
Grade 2 diarrhea (≥ 5 days) or Grade 3 diarrhea: Suspend medication and reduce dosage after recovery.
Grade 4 diarrhea or recurrence after reducing to 120mg: permanent discontinuation of medication.
2. Hepatotoxicity:
Grade 3 liver enzyme abnormality: pause until reduced after recovery.
Grade 4 liver enzyme abnormality: permanent discontinuation of medication.
3. Liver dysfunction: The initial dose for patients with severe liver injury (Child Pugh C) is reduced to 80mg.
7、 Medication precautions
1. Medication time: It should be taken with meals at a fixed time every day.
2. Gastric acid regulator:
Avoid combination therapy with proton pump inhibitors (PPIs) or H2 receptor antagonists.
Antacids should be taken every 3 hours.
3. Drug interactions:
Avoid using strong/medium acting CYP3A4 inhibitors (such as ketoconazole) or inducers (such as rifampicin) in combination.
Monitor adverse reactions of P-gp substrates such as digoxin.
8、 Medication for special populations
1. Pregnant women: May cause fetal harm, effective contraception is required during treatment and within one month after the last dose.
2. Breastfeeding period: Breastfeeding is prohibited during the treatment period and within one month after the last administration.
3. Liver dysfunction: Severe liver injury requires reduction in dosage.
4. Children: Safety and efficacy have not been established.
9、 Adverse reactions
1. Common (>5%): Diarrhea (95%), nausea (43%), abdominal pain (36%), fatigue (27%), vomiting (26%), rash (18%).
2. Serious adverse reactions:
Diarrhea (40% grade 3, 0.1% grade 4)
Hepatotoxicity (1.7% leading to discontinuation of medication)
10、 Contraindications
There are no absolute contraindications, but caution should be exercised when used for individuals known to be allergic to the ingredients.
11、 Drug interactions
1. CYP3A4 inhibitors/inducers: significantly affect the blood concentration of lenatinib and should be avoided in combination.
2. P-gp substrate: may increase the toxicity of drugs such as digoxin.
12、 Storage method
Store at room temperature (20-25 ° C) and avoid moisture.
Neratinibinformation