- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
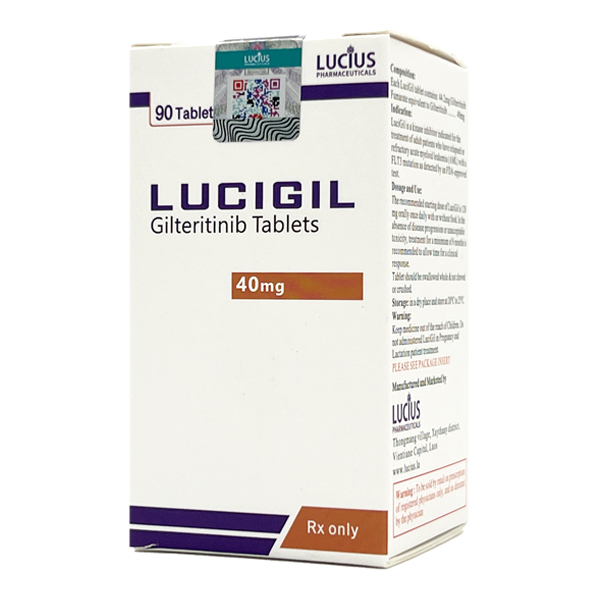
All Names: Xospata、Gilteritinib、吉瑞替尼、富马酸吉瑞替尼片、吉列替尼、适加坦
Indications:Adult patients with relapsed or refractory acute myeloid leukemia (AML)
Manufacturer:LUCIUS PHARMACEUTICAL (LAOS) CO., LTD
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Gilteritinib is an oral small molecule tyrosine kinase inhibitor that exerts anti-tumor effects by selectively inhibiting the activity of FMS like tyrosine kinase 3 (FLT3). Its mechanism of action includes blocking FLT3 receptor signaling, inhibiting leukemia cell proliferation, and inducing cell apoptosis.
1、 Drug name
1. Common name: Gilteritinib
2. Product Name: XOSPATA ®
3. English name: Gilteritinib Tablets
2、 Indications
Girotinib is suitable for the treatment of adult patients with recurrent or refractory acute myeloid leukemia (AML) confirmed by FDA approved testing methods to have FMS like tyrosine kinase 3 (FLT3) mutations.
3、 Specifications and characteristics
This product is a tablet with a specification of 40mg.
4、 Main components
Each tablet contains 40mg of active ingredient Girotinib (free base). The inactive ingredients include: mannitol, hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, magnesium stearate, hydroxypropyl methylcellulose, talcum powder, polyethylene glycol, titanium dioxide, and iron oxide.
5、 Usage and dosage
1. The recommended starting dose is once daily, 120mg per dose (i.e. 3 40mg tablets), taken orally.
2. Can be taken with food or on an empty stomach.
3. The tablet should be swallowed whole and should not be broken, crushed, or chewed.
6、 Dose adjustment
Dose adjustment is mainly based on the severity of adverse reactions. For QTc intervals greater than 500 milliseconds, it is recommended to interrupt administration and resume medication at a dose of 80mg after the QTc interval returns to within 30 milliseconds of baseline or ≤ 480 milliseconds. For pancreatitis, it is recommended to discontinue medication until the pancreatitis subsides, and then resume medication at a dose of 80mg. For other treatment-related toxicities of grade 3 or above, it is recommended to discontinue administration until the toxicity is relieved or improved to grade 1, and then resume medication at a dose of 80mg. If reversible posterior encephalopathy syndrome (PRES) occurs, the medication should be permanently discontinued.
7、 Medication precautions
1. Medication time: once a day, approximately at a fixed time, can be taken with food or on an empty stomach.
2. Omission treatment: If you miss the medication time, you should take it as soon as possible on the same day and ensure that there is at least a 12 hour interval between the next dose. The next day, resume normal medication. Do not take two doses within 12 hours.
3. Medication method: Swallow the whole tablet, do not break, crush or chew.
4. Monitoring requirements: Blood routine, blood biochemistry (including creatine kinase), and electrocardiogram (ECG) should be regularly monitored before and during treatment, and hypokalemia or hypomagnesemia should be corrected.
5. Other: Be alert to symptoms of reversible posterior encephalopathy syndrome (such as epilepsy, altered mental state), QT interval prolongation (such as dizziness, syncope), and pancreatitis (such as severe persistent abdominal pain).
8、 Medication for special populations
1. Pregnant women: Based on animal research and mechanisms of action, it may pose a risk to the fetus, and pregnant women should be informed of the potential risks.
2. Breastfeeding women: It is recommended not to breastfeed during treatment and for at least 2 months after the last dose.
3. Women and men with fertility potential: Female patients should use effective contraceptive measures during treatment and for at least 6 months after the last dose. If male patients have female partners with fertility potential, they should use effective contraceptive measures during treatment and for at least 4 months after the last dose.
4. Elderly individuals (≥ 65 years old): No overall differences in efficacy or safety were observed.
5. Liver dysfunction: Patients with mild to moderate liver dysfunction do not need to adjust the dosage. The impact on patients with severe liver dysfunction is unknown.
6. Renal insufficiency: Patients with mild to moderate renal insufficiency do not need to adjust the dosage. The impact on patients with severe renal insufficiency is unknown.
7. Children: Safety and efficacy have not yet been established.
9、 Adverse reactions
1. The most common (≥ 20%) adverse reactions include: muscle/joint pain, elevated transaminase levels, fatigue/discomfort, fever, non infectious diarrhea, dyspnea, edema, rash, pneumonia, nausea, stomatitis, cough, headache, hypotension, dizziness, and vomiting.
2. Common serious adverse reactions (≥ 5%) include pneumonia, sepsis, fever, and respiratory distress.
3. Other adverse reactions of clinical concern include: QT interval prolongation, heart failure, pericardial effusion, pericarditis, differentiation syndrome, allergic reactions, and reversible posterior encephalopathy syndrome.
4. Common laboratory abnormalities (>20%) include: elevated creatinine, hyperglycemia, hypertriglyceridemia, elevated alanine aminotransferase (ALT), elevated aspartate aminotransferase (AST), elevated alkaline phosphatase, hypocalcemia, hypoalbuminemia, elevated creatine kinase, hypophosphatemia, hypokalemia, and hyponatremia.
10、 Contraindications
Do not use for individuals allergic to gefitinib or any excipients. Allergic reactions have been observed in clinical trials.
11、 Drug interactions
1. P-gp and potent CYP3A inducers: Co administration can reduce exposure to gefitinib and may decrease efficacy, so co administration should be avoided.
2. Strong CYP3A inhibitors: Combination therapy may increase the exposure to gefitinib and alternative therapies should be considered. If it is necessary to use it together, patients' adverse reactions should be monitored more frequently, and dosage interruptions and adjustments should be made for serious or life-threatening toxicity.
3. Drugs that act on 5HT2B receptors or σ non-specific receptors (such as escitalopram, fluoxetine, sertraline): Girotinib may reduce the efficacy of these drugs and should be avoided in combination unless it is considered crucial for patient care.
12、 Storage method
Store at room temperature of 20 ° C to 25 ° C (68 ° F to 77 ° F) and allow for short distance transportation between 15 ° C to 30 ° C (59 ° F to 86 ° F). Keep the medication in its original packaging container.
Gilteritinibinformation