- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
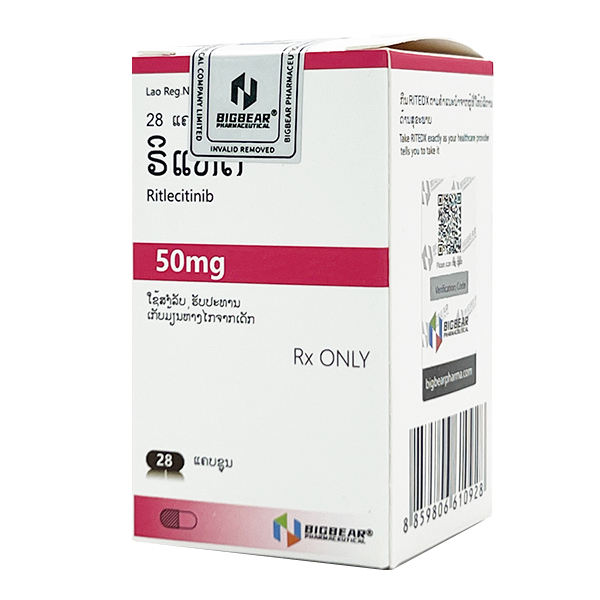
All Names: ritlecitinib、Litfulo、利特昔替尼、乐复诺
Indications:Suitable for adults and adolescents aged 12 and above with severe alopecia areata
Manufacturer:Daxiong
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Litaxitinib regulates abnormal activity of the immune system by intervening in specific signaling pathways, thereby helping to restore hair growth in affected areas.
1、 Drug name
1. Common name: Ritlecitinib
2. Product Name: LITFULO ™
2、 Indications
Litaxitinib is a kinase inhibitor suitable for the treatment of severe alopecia areata in adults and adolescents aged 12 and above.
3、 Specifications and characteristics
This product is a capsule formulation, containing 50mg of levocetinib per capsule (80.13mg based on levocetinib tosylate).
4、 Main components
1. Active ingredient: Ritlecitinib
2. Accessories: Crosslinked polyvinylpyrrolidone, glyceryl diester, lactose monohydrate, microcrystalline cellulose, hydroxypropyl methylcellulose (HPMC) capsule shell, bright blue FCF, titanium dioxide, iron oxide yellow, etc.
5、 Usage and dosage
1. The recommended dosage is 50mg orally once daily, which can be taken with food or on an empty stomach. It should be swallowed whole and should not be crushed, broken or chewed.
2. If one dose is missed, it should be taken as soon as possible. If it is less than 8 hours before the next dose, the dose should be skipped and the next dose should be taken according to the original plan.
6、 Dose adjustment
1. Liver dysfunction: Patients with mild or moderate liver dysfunction do not need to adjust the dosage; Not recommended for patients with severe liver dysfunction (Child Pugh C grade).
2. Renal insufficiency: Patients with mild to moderate renal insufficiency do not require dose adjustment; Patients with severe renal insufficiency or end-stage renal disease have not been studied yet.
3. Laboratory abnormality:
(1) If the platelet count is less than 50000/mm ³, the medication should be discontinued;
(2) If the absolute lymphocyte count (ALC) is less than 500/mm ³, treatment should be interrupted and resumed after recovery.
7、 Medication precautions
1. Before/after meals: can be taken with food or on an empty stomach, and food has no significant effect on absorption.
2. Missed medication: Take it as soon as possible. If it is less than 8 hours before the next medication, skip it.
3. Vomiting: If vomiting occurs after taking medication, it is not recommended to take the next dose according to the original plan.
4. Other: Before treatment, tuberculosis screening, viral hepatitis screening, blood routine examination, etc. are required.
8、 Medication for special populations
1. Pregnant woman: There is not enough data yet, and animal experiments have shown fetal toxicity. It is recommended to report the pregnancy status.
2. Breastfeeding period: It is not recommended to breastfeed, and breastfeeding should also be avoided within 14 hours after discontinuation of medication.
3. Children: Safe and effective for patients aged 12 and above, but there is insufficient data for patients under 12 years old.
4. Elderly people: No need to adjust dosage, but there is a higher risk of infection, so caution should be exercised when using.
9、 Adverse reactions
1. Common adverse reactions (incidence ≥ 1%) include: headache, diarrhea, acne, rash, urticaria, folliculitis, fever, atopic dermatitis, dizziness, elevated creatine kinase, herpes zoster, decreased red blood cell count, stomatitis, etc.
2. Serious adverse reactions include: severe infections, malignant tumors, cardiovascular events, thrombosis, allergic reactions, etc.
10、 Contraindications
It is contraindicated for individuals allergic to rituximab or any of its excipients.
11、 Drug interactions
1. Litacitinib inhibits CYP3A and CYP1A2. When used in combination, the blood drug concentration of relevant substrate drugs (such as certain antiarrhythmic drugs, antidepressants, etc.) should be monitored, and the dosage should be adjusted if necessary.
2. Avoid co administration with potent CYP3A inducers (such as rifampicin), as it may lead to a decrease in the blood concentration of rituximab.
12、 Storage method
Stored at 20 ° C to 25 ° C (68 ° F to 77 ° F), allowing for short distance transportation between 15 ° C to 30 ° C (59 ° F to 86 ° F). Keep the original packaging to prevent children from accidentally ingesting desiccants.
Ritlecitinibinformation