- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
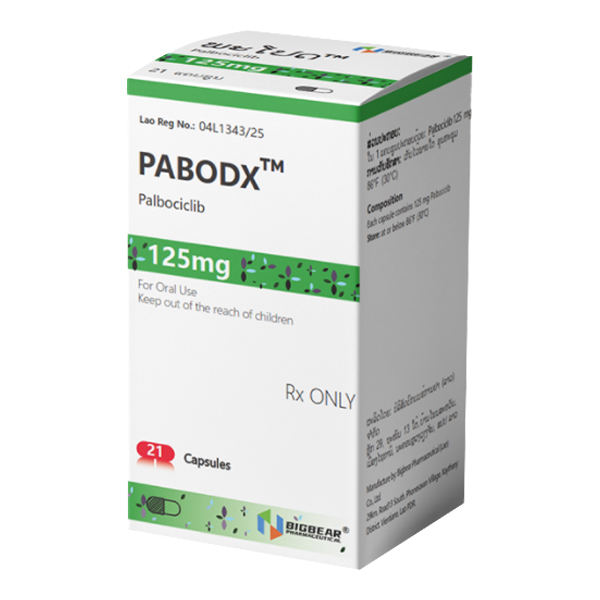
All Names: Ibrance、Palbonix、帕博西林、哌柏西利、爱博新
Indications:Suitable for the treatment of advanced or metastatic breast related diseases with hormone receptor (HR) positivity and human epidermal growth factor receptor 2 (HER2) negativity.
Manufacturer:Daxiong
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
On August 6, 2018, the National Medical Products Administration (NMPA) of China approved the listing of Pfizer's Pembrolizumab in China. This is the first approved CDK4/6 inhibitor in China and was included in the national medical insurance catalog in October 2021.
1、 Drug name
1. Common name: Pabosini
2. Product Name: IBRANCE
3. English name: Palbociclib
2、 Indications
This product, combined with letrozole, is suitable for the treatment of postmenopausal women with estrogen receptor positive and human epidermal growth factor receptor 2 negative advanced breast cancer, as the initial endocrine treatment plan for their metastatic diseases.
3、 Specifications and characteristics
125mg capsules: opaque hard gelatin capsules.
4、 Main components
1. Active ingredient: Pabosini.
2. Accessories: Microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, colloidal silica, magnesium stearate, and hard gelatin capsule shell. The capsule shell contains gelatin, iron oxide red, iron oxide yellow, and titanium dioxide; Printing ink contains cordyceps, titanium dioxide, ammonium hydroxide, propylene glycol, and silicone oil.
5、 Usage and dosage
1. Recommended starting dose: 125mg, once daily, taken with meals.
2. Medication plan: Take continuously for 21 days, then stop taking for 7 days, forming a complete 28 day treatment cycle. This product needs to be used in combination with letrozole (2.5 milligrams, once daily, taken continuously within a 28 day cycle).
3. Usage: The capsule should be swallowed whole and should not be chewed, crushed, or opened. If the capsule is damaged, cracked, or incomplete, it should not be taken.
6、 Dose adjustment
1. Dose adjustment is based on individual safety and tolerability.
2. Recommended dosage level: Initial dose of 125 milligrams per day; Reduce the initial dose to 100 milligrams per day; The second dose was reduced to 75 milligrams per day. If it needs to be reduced to below 75 milligrams per day, treatment should be stopped.
3. Hematological toxicity adjustment: Based on indicators such as neutrophil count, it may be necessary to suspend administration, delay the start of the next cycle, and/or reduce dosage.
4. Non hematological toxicity adjustment: For persistent grade 3 or higher non hematological toxicity, administration should be suspended until symptoms improve, and then treatment should be resumed at a reduced dose.
5. Combination use with strong CYP3A inhibitors: Combination use should be avoided. If combined use is necessary, the dosage of this product should be reduced to 75 milligrams once a day; After discontinuing potent inhibitors, the original dosage can be restored.
7、 Medication precautions
1. Medication time: Take with meals at approximately the same time each day to increase absorption and reduce fluctuations in blood drug concentration.
2. Omission or vomiting: If the patient vomits or misses one dose, it should not be taken on the same day. The next prescription dose should be taken at the regular time.
8、 Medication for special populations
1. Pregnant women: Based on animal research and its mechanism of action, this product may cause fetal harm and is contraindicated for pregnant women. Women of childbearing age should take effective contraceptive measures during treatment and for at least two weeks after the last dose.
2. Breastfeeding women: It is recommended that breastfeeding women stop breastfeeding during the treatment period.
3. Male of childbearing age: Based on animal findings, treatment may impair male fertility.
4. Children: Safety and efficacy have not yet been determined.
5. Elderly individuals: No overall differences were observed compared to younger patients, but it cannot be ruled out that some elderly individuals have higher sensitivity.
6. Liver/kidney dysfunction: Patients with mild liver injury or mild to moderate kidney injury do not require dose adjustment. There is a lack of data on patients with moderate or severe liver injury and severe kidney injury.
9、 Adverse reactions
1. The most common adverse reactions (with an incidence rate of ≥ 10%) include: neutropenia, leukopenia, fatigue, anemia, upper respiratory tract infections, nausea, stomatitis, hair loss, diarrhea, thrombocytopenia, decreased appetite, vomiting, weakness, peripheral neuropathy, and nosebleeds.
2. Serious adverse reactions to be cautious of: neutropenia (requiring close monitoring of blood routine), infection, pulmonary embolism.
10、 Contraindications
There are currently no obvious contraindications, but patients should be cautious to prevent allergies to drug ingredients.
11、 Drug interactions
1. Strong CYP3A inhibitors: Avoid simultaneous use. If unavoidable, the dosage of this product should be reduced.
2. Strong and moderate CYP3A inducers: Avoid using them simultaneously.
3. Sensitive CYP3A4 substrates with narrow therapeutic window: Co administration with this product may require reducing the dosage of these drugs.
4. Grapefruit products: may increase the blood concentration of this product, and should be avoided during treatment.
12、 Storage method
Store at 20 ℃ to 25 ℃ (68 ℉ to 77 ℉); Allow short distance transportation between 15 ℃ and 30 ℃ (59 ℉ and 86 ℉).
Palbonixinformation