- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
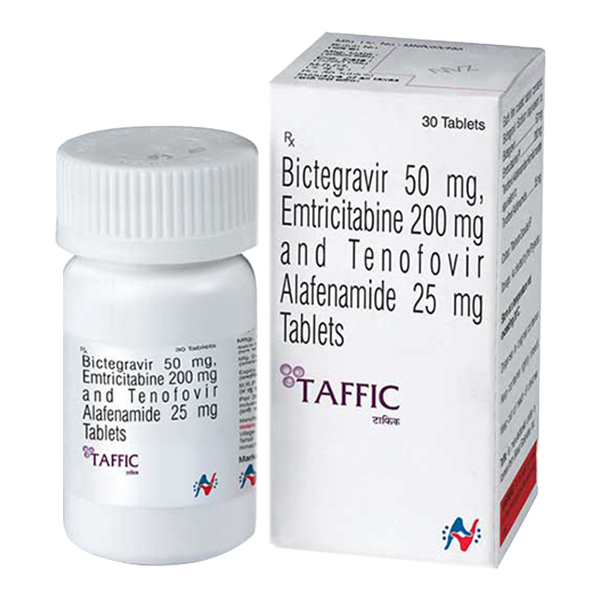
All Names: Biktarvy、bictegravir/emtricitabine/tenofovir alafenamide、必妥维、比克恩丙诺片
Indications:Children and adults infected with AIDS weighing at least 14kg
Manufacturer:Indian Sitro Pharmaceuticals
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Bituximab is a compound antiviral preparation containing the integrase inhibitor Bicentecavir (BIC), nucleoside reverse transcriptase inhibitor Entricitabine (FTC), and propafenone (TAF).
1、 Drug name and main ingredients
1. Common name: Bictegravir/Emtricitabine/Tenofoviralafenamide
2. Product Name: Biktarvy ®
3. Dosage form: Film coated tablets
4. Main ingredients: Each tablet contains 52.5mg of bikinamivir sodium (equivalent to 50mg of bikinamivir), 200mg of emtricitabine, and 28mg of propafenone (equivalent to 25mg of tenofovir). Auxiliary materials include cross-linked carboxymethyl cellulose sodium, magnesium stearate, etc.
2、 Indications
HIV-1 infection: Used to treat adult HIV-1 infection, suitable for the following two types of patients:
Patients who have not received antiretroviral therapy;
Patients with virological suppression (HIV-1 RNA<50 copies/mL) and no history of treatment failure can replace the current stable regimen (requiring continuous medication for ≥ 3 months).
3、 Specifications and characteristics
1. Specifications: Each tablet contains 50mg of bikinamivir, 200mg of emtricitabine, and 25mg of tenofovir.
2. Appearance: Capsule shaped film coated tablets.
4、 Usage and dosage
1. Recommended dosage: 1 tablet per day, oral, can be taken with food or on an empty stomach.
2. Omission treatment: If missed, it should be replenished as soon as possible; If it is close to the next medication time (within 4 hours), skip the missed dose.
3. Vomiting treatment: Vomiting after taking medication does not require supplementation, and the next medication should be taken according to the original plan.
5、 Dose adjustment
1. Renal insufficiency: It is not recommended to use it for patients with creatinine clearance rate (CrCl)<30mL/min.
2. Liver dysfunction: Not recommended for Child Pugh C (severe) patients.
6、 Medication precautions
1. Dietary impact: Not affected by food, but avoid taking with antacids containing aluminum/magnesium/calcium (with a 2-hour interval).
2. HBV co infection: Monitor the risk of hepatitis recurrence after discontinuation of medication.
3. Drug interactions: Combination use of digoxin and rifampicin is prohibited.
7、 Medication for special populations
1. Pregnant women: It is necessary to weigh the pros and cons, and medication used during pregnancy needs to be registered (APR registration number 1-800-258-4263).
2. Breastfeeding period: Breastfeeding is prohibited.
3. Children: Safety has not been established for those under 18 years old.
8、 Adverse reactions
1. Common (≥ 5%): Diarrhea (6%), nausea (5%), headache (5%).
2. Serious reactions: Immune reconstitution syndrome (5.3%), lactic acidosis (rare).
9、 Contraindications
1. Combining digoxin or rifampicin;
2. People who are allergic to ingredients.
10、 Drug interactions
1. CYP3A strong inducers (such as rifampicin): reduce the concentration of bikinamivir and prohibit combination use;
2. OCT2/MATE1 substrates (such as metformin): may increase their blood drug concentration.
11、 Storage method
1. Original packaging preservation, moisture-proof, built-in desiccant;
2. Original packaging preservation, moisture-proof, built-in desiccant; Storage temperature:<30 ℃.
Note: During treatment, regular monitoring of renal function, HBV markers, and HIV viral load is required. After missing or vomiting, there is no need to supplement the medication, but it is necessary to strictly follow the daily dosing plan.
Biktarvyinformation