- All Drug Categories
- Home
- Anti-cancer Drugs
- Healthcare Products
- Medical Information
- Statement
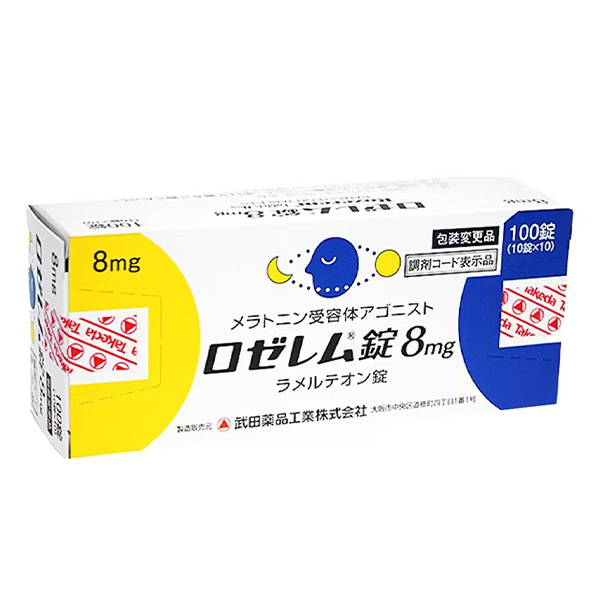
All Names: Ramelteon、Rozerem、雷美替胺、雷美尔通、瑞美替昂、拉米替隆
Indications:Adult insomnia patients who have difficulty falling asleep.
Manufacturer:Takeda,Japan
Customs Clearance Procedure:If the customs requires the package for customs clearance, please pay the customs clearance fee according to the content of EMS SMS and customs regulations.
Remdesidine is a selective melatonin receptor agonist developed by Takeda Pharmaceuticals in Japan.
1、 Drug name
Common name: Ramelteon
Product Name: Lozeren Tablets 8mg (Lozerem Tablets 8mg)
2、 Indications
Used to improve insomnia and difficulty falling asleep.
3、 Specifications and characteristics
Specification: 8 milligrams per tablet.
Appearance: This product is a film coated tablet. The color is light reddish yellow. Round tablets, with a diameter of 7.1 millimeters, a thickness of 3.6 millimeters, and a mass of 135 milligrams.
4、 Main components
Active ingredient: Each tablet contains 8 milligrams of remitinide.
5、 Usage and dosage
Conventional usage: Usually 8 milligrams per dose for adults, taken orally before bedtime.
Key tip: It must be taken immediately before bedtime. If it is necessary to get up during sleep to work after taking it, it should not be taken.
6、 Dose adjustment
Elderly patients: Due to the possible increase in blood drug concentration, medication should be administered with caution based on the patient's condition.
Patients with liver dysfunction:
Patients with severe liver injury: Do not use.
Mild to moderate liver injury patients: Blood drug concentration may increase and should be used with caution.
Patients with renal insufficiency: Patients with mild to severe kidney injury and chronic hemodialysis have limited experience in using it, and no significant differences have been observed compared to healthy adults.
7、 Medication precautions
Medication time:
Take it immediately before bedtime.
Postprandial administration may lead to a decrease in blood drug concentration, and should be avoided when taken with meals or immediately after meals. It is recommended to take on an empty stomach or before bedtime.
Omission/vomiting: The information is not explicitly mentioned. Considering that this medicine is used before falling asleep, if the medication time has already been missed, it should not be taken in the middle of the night.
Driving and operating machinery: After taking medication, there may still be drowsiness, decreased attention/concentration/reflex movement ability the next day, so driving a car or operating dangerous machinery is prohibited.
Treatment cycle: After 2 weeks of treatment, the effectiveness and safety should be evaluated. If there is no effect, consider stopping the medication to avoid long-term blind medication.
8、 Medication for special populations
Pregnant women: Use only when the benefits of treatment outweigh the potential risks. Animal experiments have reported teratogenicity.
Breastfeeding women: The benefits of breastfeeding should be balanced against the necessity of treatment. Animal test reports show that drugs can enter breast milk.
Children: Lack of clinical data to establish its effectiveness and safety.
Elderly people: Medication should be administered with caution as blood drug concentrations may increase.
Patients with sleep apnea syndrome: Severe patients have insufficient experience in using it, and safety has not been established.
9、 Adverse reactions
Common adverse reactions (incidence ≥ 0.1% to<5%): dizziness, headache, drowsiness, constipation, nausea, fatigue, etc.
Occasional or unknown frequency of adverse reactions: nightmares, rash, elevated prolactin, suicide attempts, etc.
Serious side effects (frequency unknown): Allergic shock (urticaria, angioedema, etc.).
10、 Contraindications
Patients with a history of allergies to the ingredients of this medication.
Patients with severe liver dysfunction.
Do not use in combination with fluvoxamine as it significantly increases the blood concentration of this drug.
11、 Drug interactions
Prohibition of combination use: Fluvoxamine (a strong CYP1A2 inhibitor) can significantly enhance the efficacy of this drug.
Caution should be exercised when using:
CYP1A2 inhibitors (such as certain quinolone antibiotics): may enhance the efficacy of this drug.
CYP2C9 inhibitors (such as fluconazole and other azole antifungal drugs): may enhance the efficacy of this drug.
CYP3A4 inhibitors (such as macrolide antibiotics, ketoconazole, etc.): may enhance the efficacy of this drug.
CYP inducers (such as rifampicin): may weaken the effect of this drug.
Alcohol: Combined use with alcohol may enhance central inhibitory effects such as decreased attention and concentration.
Other interactions (such as combination with fluoxetine, gabapentin, omeprazole, etc.) may affect pharmacokinetics, please refer to the "Interactions" section of the instruction manual for details.
12、 Storage method
Store at room temperature. The validity period is 3 years.
13、 Manufacturer
Production and sales enterprise: Takeda Pharmaceutical Industry Co., Ltd.
Ramelteoninformation